Abstract
Background: Venetoclax-based regimens have become a standard of care for newly diagnosed (ND) unfit acute myeloid leukemia (AML) patients and are also routinely used in the relapsed/refractory (R/R) AML or myelodysplastic syndrome with excess blasts-2 (MDS-EB2) settings. Herein, we report our real-life experience with Venetoclax based regimens for the treatment of AML or MDS-EB2.
Aims: To evaluate the outcomes of AML or MDS-EB2 patients treated with Venetoclax-based regimens.
Methods: This was an observational, single-center, retrospective study. The patients were older than 18 years of age, had either AML or MDS-EB2 and were ND or R/R. All patients provided informed consent for treatment and data collection. The treatment regimens consisted of Venetoclax in combination with either chemotherapy (Venetoclax 600mg/d with low dose Cytarabine 20mg/m2 D1-10 and/or Actinomycin D 12.5mcg/kg D1-3), or hypomethylating (HMA) agents (Venetoclax 400mg/d with Decitabine 20mg/m2 D1-5/D1-10 or Azacitidine 75mg/m2 D1-7). The number of Venetoclax days per cycle was adapted individually based on the early bone marrow evaluations and toxicity but could not exceed 28 days per cycle. The cycles were administered every 28-42 days depending on the count recovery and patient's status. Patients with targetable mutations (FLT3, N(K)RAS, BCR-ABL1) received concomitant FLT3-inhibitors, Trametinib or BCR-ABL1-inhibitors. IDH-inhibitors were not used. Responders could proceed to either allogeneic stem cell transplantation (alloSCT) or continue Venetoclax based therapies as maintenance based on their status and genetic risk factors. We evaluated baseline characteristics, composite CR (CRc = CR + CRi + CRp), overall response (ORR = CRc + MLFS), MRD negativity rates (<0.1% by multiparameter flow cytometry), overall survival (OS), predictors for OS, relapse-free survival (RFS) for responders, day 30, day 60 mortality rates.
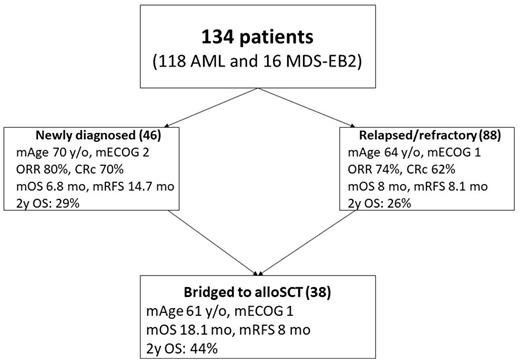
Results: 134 patients had been included in the study of whom 118 (88%) had AML and 16 (12%) had MDS-EB2. Sixty eight patients (51%) were female, the median age was 66 (20-88) years and the median ECOG was 2 (0-4). One third (34%, 46/134) of cases were ND, whereas 66% (88/134) had R/R disease. Secondary AML or MDS-EB2 was diagnosed in 52% (70/134) of patients. Adverse karyotype was found in 38% (51/134) of cases. 75% (101/134) of patients were treated with Venetoclax + chemotherapy and 25% (33/134) received Venetoclax + HMA. Treatment responses were evaluated in 125 patients. The ORR was 76% (95/125). The CRc rate was 65% (77/119), after exclusion of 6 MLFS patients who proceeded to sequential alloSCT in aplasia. The ORR and CRc rates did not significantly differ between ND (80% (32/40) and 70% (28/40)) or R/R (74% (63/85) and 62% (49/79)) patients, respectively, p>0.05. 44% (34/77) of CRc patients had achieved MRD negativity. The highest ORR was seen in patients with TET2 (100%, 15/15), GATA2 (100%, 5/5), IDH2 (95%, 21/22), NPM1 (90%, 19/21), DNMT3A (90%, 19/21), (N/K)RAS (89%, 16/18), FLT3 (88%, 22/25) and IDH1 (86%, 12/14) mutations. Of note, 100% (11/11) of patients with concomitant NPM1 and IDH1/2 mutations had responded with a 73% (8/11) MRD negativity rate, indicating very high efficacy of Venetoclax-based therapies in this genetic subgroup. The lowest ORR was observed in patients with adverse karyotypes (57%, 27/47), TP53 (56%, 9/16) and PTPN11 (56%, 5/9) mutations. 28% (38/134) had been bridged to alloSCT. The median OS was 8 months (6.6-10.8) for all patients (6.8 and 8 months for ND and R/R patients, respectively, p=0.649). The median RFS was 9.7 months (7.8-33.6) for all patients (14.7 and 8.1 months for ND and R/R patients, respectively, p=0.285). In a univariable analysis IDH2, DNMT3A mutations, and bridging to alloSCT significantly improved OS, whereas ECOG 3-4, secondary disease, PTPN11, TP53 mutations, and adverse karyotype were associated with worse OS. In a multivariable analysis ECOG 3-4, TP53, and PTPN11 mutations were associated with worse OS, whereas DNMT3A, IDH2 mutations, and alloSCT improved OS. Day 30 and day 60 mortality rates were 9% (12/134) and 18% (24/134), respectively.
Conclusions: Venetoclax based regimens produce high remission rates in both ND and R/R AML or MDS-EB2 in the real-life setting. Poor performance status, TP53 and PTPN11 mutations negatively impact OS, whereas DNMT3A, IDH2 mutations and bridging to alloSCT are associated with improved OS.
Disclosures
Žucenka:Abbvie: Consultancy, Honoraria, Other: Travel Expenses; Astellas: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Other: Travel Expenses; Pfizer: Consultancy. Pileckytė:Abbvie: Consultancy, Honoraria. Griškevičius:Miltenyi Biomedicine: Membership on an entity's Board of Directors or advisory committees.
OffLabel Disclosure:
Venetoclax - for R/R AML and MDS-EB2 treatment
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal